What are Aroma Chemicals?
Naturally when most of us outside the world of perfumery or the chemical industry hear the word "chemical" then we think "not natural, toxic, dangerous". When we hear the words "natural" we think, "safe, healthy". Of course the chemists among us know that everything around us is made up of chemicals. Water is a chemical, vegetables are complex mixtures of structured chemicals, wood is made up of cellulose, a chemical and the Earth is one big bundle of chemicals. We eat chemicals, we drink chemicals, we are chemicals ourselves. So now, I try to ask you to put these prejudices on hold for a while. When we extract essential oils from plants by distillation what we are doing is heating up the smaller volatile chemicals held in the plant material, cellulose
| For example:
|
If these chemical names do not seem to be that foreboding that is because in perfumery the 'trivial' names used are reflected in where that chemical has been discovered in nature. When the chemicals were discovered, chemists may not have known the chemical structure, or they thought the name more appropriate. So for example Linalool was first discovered as the main component in the essential oil from Linaloe berries, chemists knew it was an alcohol (r- OH) and so added an "ol" (suffix for alcohols) to the name Linaloe+ol = Linalool - the alcohol found in Linaloe.
You will note that when we use an essential oil in a perfume we are adding all of its component chemicals, the good and the bad, and this is where the secret of the success of the aroma chemical lies.
When nature grows a plant each individual plant has a slightly different make up in its DNA and produces a slightly different mixture in the essential oil, the plants efficiency is varied by the soil quality, the amount of sunshine, water, wind etc. And so every year the oil composition changes maybe just a little but it is never exactly the same. We live the in age of the McDonalds, consumers expect to get the same taste wherever they buy their Big Mac. Likewise with perfumes, our public demand the same smell every time. Nature doesn't play like this. In a single bag of apples, can you find two exactly the same? Additionally, oils frequently contain some undesirable qualities that we don't want, such as traces of toxins (e.g. bergaptene
![]() in cold pressed citrus oils). Now if we could extract these offending chemicals from an oil, we could improve its properties.
in cold pressed citrus oils). Now if we could extract these offending chemicals from an oil, we could improve its properties.
In Lemon Oil d'limonene makes up over 90% of the oil, the problem is that d'limonene despite being the major ingredient adds little to the overall odour as it has a very soft odour
![]() . Additionally the d'limonene has poor solubility in alcohol and water mixtures and in the presence of air (oxygen) polymerises
. Additionally the d'limonene has poor solubility in alcohol and water mixtures and in the presence of air (oxygen) polymerises
![]() to form a thick resinous substance. So when making a fine fragrance we may use a lemon oil with d'limonene removed by fractional distillation
to form a thick resinous substance. So when making a fine fragrance we may use a lemon oil with d'limonene removed by fractional distillation
![]() (terpeneless lemon oil) to get a more soluble and stable product. The d'limonene extracted can then be used as a solvent, in cheaper household fragrances or, in theory as a starting material for the production of other aroma chemicals through a series of reactions.
(terpeneless lemon oil) to get a more soluble and stable product. The d'limonene extracted can then be used as a solvent, in cheaper household fragrances or, in theory as a starting material for the production of other aroma chemicals through a series of reactions.
So, the first source of aroma chemicals is from isolates of essential oils.
The main source of aroma chemicals from isolates is from pinene (alpha & beta) which can be fractionally distilled from turpentine oil or oils from a number of species of pine. However, now in practice, the main source is a by-product of the paper industry. When paper is produced from wood pulp the essential oil is not wanted and in times-gone-by being a volatile substance was a pollutant released into the air. In an attempt to reduce these pollutants the paper manufacturers got together with the aroma chemical manufacturers.
The second source is chemically modified isolates from essential oils.
The chemists found a way to capture the alpha-pinene which they could then process into many other aroma chemicals. The alpha pinene is converted to its isomer beta-pinene and then undergoes a series of chemical reactions from which Myrcene, Linalool, Citral and many other materials are produced.
The third source of aroma chemicals is from the petrochemical industry.
Chemists use fractions of crude oil such as Benzene, Toluene and Xylene. These basic chemicals undergo a series of chemical modifications to produce Phenyl Ethyl Alcohol, Benzyl Acetate, Musk Xylol, para-Tolylaldehyde and a myriad of other aroma chemicals.
These chemicals are often found in essential oils, Phenyl Ethyl Alcohol in Rose, Benzyl Acetate in Jasmin and may be labelled "occurs in nature" or "nature identical" but others such as the Musk Xylol are entirely synthetic.
Some materials such as Linalool can be obtained by all three processes
1) linalool separated from Bois de Rose (Rosewood) Oil
2) linalool synthetic from the pinene route beta-pinene > myrcene > linalool
3) via a patented Roche process from benzene (not normally used)
It should be noted that each source of Linalool gives a slightly different product and therefore a slightly different odour as the molecule can exist as a d- dextro
![]() or l- leavo
or l- leavo
![]() isomer which occur in different ratios and other trace impurities will be present from the specific manufacturing process.
isomer which occur in different ratios and other trace impurities will be present from the specific manufacturing process.
1) From Bois de Rose Oil - l-Linalool - has a light chemical floral woody fresh odour
2) From Coriander Oil - d-Linalool - has a heavier chemical woody slightly hot odour.
3) Synthetic Linalool is a mixture of the isomers d,l-Linalool, the balance depending on the process - light chemical woody fresh.
Synthetic Linalools don't differ greatly in their odour, hence their choice as the reference material for the relative odour impact of materials. The natural forms are a different story depending not only on the starting material but also each producers precise manufacturing process. It is difficult to overuse Linalool synthetic in almost any fragrance and 30% or more can be incorporated in a formula. However, some natural source Linalools, particularly the d isomer from Coriander must be restricted to just 1 or 2 %.
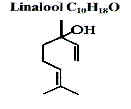
| The reason we asked you to suspend any prejudice you may have about chemicals is that, when novices start to mix perfumes for the first time they are frequently reluctant to use aroma 'chemicals'. Yes, chemicals should be treated with care, but, not any more than the care that should be exercised with the natural essential oils. Overall, there are probably more hazards in many pure essential oils than there are in most aroma chemicals. |
| Natural Oils |
|
||
|---|---|---|---|
|
|
|
|
|
| Natural | Variable quality | Consistent quality | Poor consumer perception |
| Complex odours that add depth | Some sources non-renewable | Generally stable prices
(Decreasing in real cost) | Dependent on supply of source materials which are only by-products of much larger industries (viz. paper and oil) |
| Superior pharmacological activity | May contain toxins or chemicals - if synthetic would be banned from flavours or perfumes | Interaction with product bases or other ingredients can be controlled. | Some industrial quality chemicals sold as food or cosmetic grades |
| Components responsible for interaction and changes in colour and odour hard to remove | Simple clearer, controllable odours | ||
| Limited quantities when upscaling production | |||
| Labour and land intensive | |||
| Supply subject to natural cycles and phenomena | |||
| Price varies and regularly increases in comparison to synthetics | |||