Alcohols
"Alcohol" is commonly known as the alcohol we drink in beer, wine and spirits and the solvent used in perfumes and colognes. But you've probably already seen names such as Benzyl Alcohol, Phenyl Ethyl Alcohol.

|

|
From a chemists point of view, "alcohols" really refer to functional groups in a chemical's molecule.
A chemical molecule is made up of a radical (the main body)

|
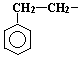
|
and functional groups (the arms and legs).
|
|
|
It is generally the functional groups that have the most effect on the properties of a chemical or its smell as these are the parts that reach out to interact with other molecules or indeed the smell receptors in our nose. The alcohol group is the OH in the molecule. This group is polar, i.e. likes water.
The smallest Molecule with an alcohol group is Methyl Alcohol.
Ethyl Alcohol is the next one up in size, this is the one we know as "alcohol", "ethanol", "grain alcohol".
Propyl Alcohol has 3 carbons ( CH 3- CH 2 -CH 2 -OH ). You will commonly come across iso-propyl alcohol (the iso refers to a slight difference in arrangement of the atoms in the molecule) which has good solvent properties but the smell is a little too strong for fine perfumery. It does however find use in air freshener bases and as a cleaning solvent as it is usually duty free
Butyl Alcohol has 4 carbons ( CH 3 -CH 2 -CH 2 -CH 2 -OH ). We come across traces of this in beer and whisky and is said to be responsible for hangovers. It is used in traces in flavours and fragrances.
The Carbon radicle forms a non-polar (doesn't like water) section of the molecule. The combination of the small oily radicle and the polar functional group give these small molecules the ability to mix with water AND volatile oils.
These are examples of straight-chain (aliphatic) alcohols but we can often recognize them as the Alcohol functional group is generally indicated in a chemicals name (nomenclature) by adding an OL on the end. Such as these names Linalool, Geraniol, Hexenol, Terpineol, Eugenol, Phenol.
The polarity of the alcohol group leads to chemicals tending to have softer smells when compared to similar structured chemicals with other functional groups like aldehydes or esters. Molecules with alcohol groups also tend be be more stable than esters or aldehydes in applications (end products). We will look at these other functional groups in later articles.
| Note that the alcohol group is usually indicated in short hand form with an OL at the end of the chemicals name.
eg. |